PDS Flexible Plates are thin, bendable sheets used mainly in facial or cranial surgeries to help support and shape healing bones or soft tissues.
“PDS” stands for Polydioxanone, which is a biodegradable polymer. This means it slowly dissolves in the body over time, so there’s no need to remove it later with another surgery.
Unlike metal plates (like titanium mesh), PDS flexible plates are soft and flexible, making them useful for delicate areas, especially in children or in areas like the nose, eye sockets, or jaw.
They are often trimmed and shaped during surgery to fit the specific area.
In simple terms:
PDS Flexible Plates are like soft, dissolvable bandages made of special plastic that help bones and tissues in the face heal properly. They are safe, don’t need to be taken out later, and are gentler than metal plates.
How do PDS plates work, and how long do they stay in the body?

PDS plates are ultra‑thin, bioabsorbable sheets crafted from the same polydioxanone polymer as PDS™ sutures. In procedures like septorhinoplasty or orbital‑floor reconstruction, they’re anchored temporarily to support and reshape cartilage or soft bone.
While in place, they provide mechanical reinforcement and a scaffold that promotes new tissue growth. As healing advances, they slowly hydrolyze, transferring the load to the regenerated tissue until the plate is completely absorbed.
Advantages and Disadvantages of PDS Cranial Flexible Plates
| Advantages | Disadvantages |
|---|
| Absorbable – No need for removal surgery | Less strong than metal plates (e.g., titanium) |

| Flexible – Easily shaped to fit complex areas | May lose strength before full healing occurs |
| Biocompatible – Low risk of rejection | Not suitable for high-load bearing areas |
| Reduces long-term foreign material in body | Can be more expensive than some permanent options |
| Ideal for pediatric use or growing bones | Limited use in very complex or large fractures |
| Helps avoid growth problems in children | May degrade faster in some body environments |
| Reduces risk of long-term infection | May not be visible on X-rays once absorbed |
Table outlining the advantages and disadvantages of PDS Flexible Plates.
Pros and Cons of PDS Flexible Cranial Plates

| Pros in Surgery | Cons in Surgery |
| Absorbable – avoids second surgery to remove plate | Limited mechanical strength – not ideal for load-bearing areas |
| Flexible – easy to trim and shape during surgery | Degradation timeline may not match bone healing in all cases |
| Biocompatible – reduces inflammation and rejection | Not suitable for complex or large fractures |
| Ideal for pediatric surgeries – supports growing bones | Requires precise placement to avoid early degradation |
| Minimizes long-term foreign body presence | May not provide enough long-term support in adults |
| Useful in delicate facial and cranial areas | Reduced visibility in imaging once absorbed |
PDS Flexible Plates are made from
- Made from polydioxanone (PDS) – a biodegradable polymer used in dissolvable sutures.
- Flexible because of its soft, plastic-like structure (not metal).
- Composed of a polymer matrix – chains of molecules that allow bending without breaking.

- Can be warmed and shaped during surgery to fit the surgical site.
- Holds its shape once cooled, providing temporary support.
- Gradually absorbed by the body over time, eliminating the need for removal.
- Non-metallic – unlike memory alloys like nitinol, it doesn’t have shape memory but remains moldable.
- Safe and biocompatible, ideal for delicate or growing tissue (e.g., in children).
Ingredients (Material Composition) of PDS Flexible Plates:
- Polydioxanone (PDS):
- A synthetic polymer made from p-dioxanone monomers.
- It is the primary and only active material in PDS plates.
- Belongs to the polyester family of polymers.
- Known for being biodegradable, absorbable, and biocompatible.
👉 No metal, no memory alloy, and no mixed materials.
👉 It is manufactured in a sterile, medical-grade form for surgical use.

Preparation For PDS Flexible PLATES

Ready for Use When:
- Properly sized and shaped.
- Softened if needed.
- Maintains sterility throughout preparation.

Polydioxanone bioabsorbable implant
Resorbable fixation plate
Perforated polymer surgical plate
0.15 × 50 × 40 mm PDS plate
Special post‑op care or activity restriction required when using PDS plates?
| Recommendation | Duration |
|---|---|
| Wound care • Keep incision sites clean and dry • Change dressings as instructed | Until sutures are removed (≈7–10 days) |
| Head elevation • Sleep with head elevated (pillows or recliner) to reduce swelling | First 5–7 days |
| Cold compresses • Apply cold packs (wrapped) 10 min on / 10 min off to lessen bruising/swelling | First 48 hours |
| Strenuous activity • No heavy lifting, bending, or vigorous exercise | 2–3 weeks |
| Facial movements • Avoid blowing your nose, wide yawning, or chewing very hard | 1–2 weeks |
| Contact sports • No helmets, goggles, or any direct‑impact activities | 6–8 weeks |
| Medications • Take prescribed antibiotics and pain relievers as directed | As prescribed |
| Follow‑up visits • Attend post‑op checkups to monitor healing and plate absorption | Surgeon’s schedule |
Tip: Always follow your surgeon’s specific instructions—individual healing times and restrictions can vary.
6 Medical Conditions PDS Plate is Use for
Septorhinoplasty & Nasal Septum Reconstruction
Serve as a scaffold to reinforce columellar struts, L‑struts, septal extension grafts and to reconstruct or reshape the nasal septum in cosmetic and functional rhinoplasty procedures.
Alar Batten & Lateral Crural Strut Grafts
Support weak lower‑lateral (alar) cartilages by creating batten grafts or lateral crural struts, enhancing tip projection and contour without harvesting additional autologous tissue.
Nasal Septal Perforation Repair
Act as an underlay or overlay scaffold—often combined with fascia grafts—to close septal perforations and promote mucosal healing, achieving high closure rates with minimal complications.
Nasoseptal Fracture (Open Septal Reduction)
Used as an internal stent to reinforce and realign fractured septal cartilage in open reduction of septal fractures, improving stability and contour during healing.

Endoscopic Skull Base & CSF Leak Repair
Provide a rigid, bioabsorbable barrier for multilayer closure of skull‑base defects (e.g., encephaloceles or cerebrospinal fluid leaks) via endonasal endoscopic approaches.
General Maxillofacial & Plastic Surgery Reconstruction
Employed to reinforce thin cartilage or bone frameworks in facial contour corrections (e.g., orbital floor, malar augmentation) and other craniomaxillofacial procedures, offering temporary support before gradual resorption.
Contraindications (Who Should NOT Use PDS Flexible Plates):
DS Flexible Plates are generally safe, but they are not suitable for everyone or every surgical situation. Here’s a list of who should not use them:
🚫 1. Patients with Heavy Load-Bearing Needs
- Not suitable for areas that require strong mechanical support (e.g., jaw reconstruction, weight-bearing cranial bones).
- PDS is not as strong as metal plates and can degrade before full healing in high-stress zones.
🚫 2. Severe or Complex Fractures
- In cases with large or comminuted fractures, especially in the orbit or midface, the plate may not provide enough long-term support.
🚫 3. Allergy or Sensitivity to Polymers
- Although rare, patients with known allergies to synthetic absorbable polymers (polydioxanone) should avoid use.
🚫 4. Poor Wound Healing or Infections
- Not recommended in infected surgical fields or where tissue healing is compromised (e.g., radiation damage, poor blood supply).
🚫 5. Adults Needing Permanent Structural Support
- Because PDS is absorbable, it may not be appropriate in older adults or those who require permanent fixation.
🚫 6. Areas Requiring Radiographic Visibility
- Once absorbed, the plate cannot be seen on X-rays, which may be a drawback in follow-up imaging or trauma assessment.

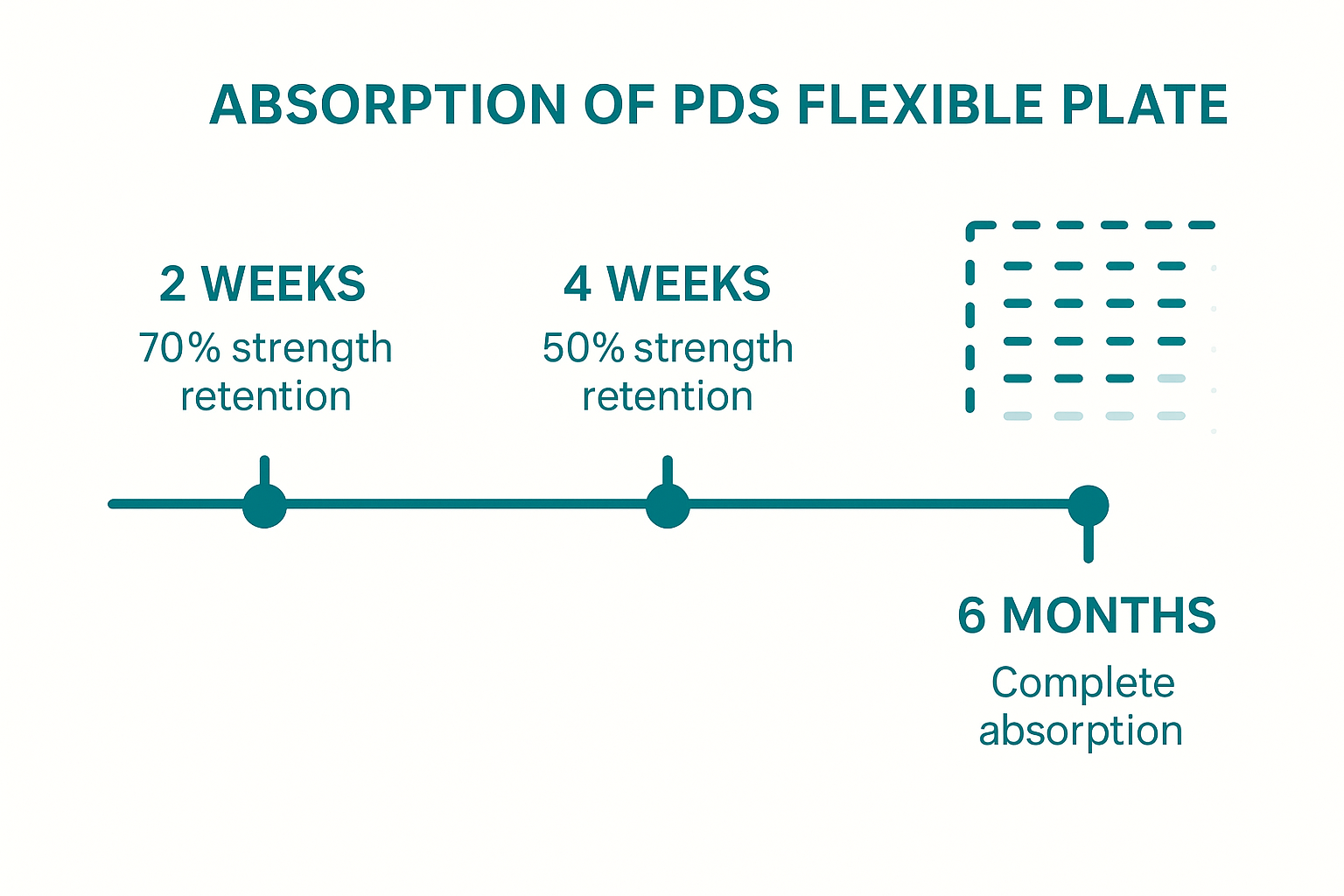
⏳ Absorption Time of PDS Flexible Plate (Polydioxanone)
- Initial strength retention:
- Around 70% at 2 weeks,
- 50% at 4 weeks,
- Drops significantly by 6 weeks.
- Complete absorption:
- Typically occurs within 6 months (approximately 180 days).
- Absorption happens through hydrolysis (breaking down in the presence of body fluids).
✅ PDS Flexible Plates provide temporary support for healing tissues, then gradually dissolve within 6 months, reducing the risk of long-term foreign body complications.
✅ FDA Approval:
- Regulated as a Class II medical device under the FDA.
- Listed in the FDA’s Global Unique Device Identification Database (GUDID) under Ethicon (a Johnson & Johnson company).
- Labeled for absorbable internal use in soft tissue and bone support, especially in craniofacial reconstruction.
✅ CE Mark (Europe):
- PDS plates distributed in Europe carry a CE mark, meaning they meet the EU’s Medical Device Regulation (MDR) standards.
- They are certified for use in craniofacial, nasal, and orbital repairs in both pediatric and adult populations.
Is PDS plates an Implant
Yes. PDS (polydioxanone) plates are indeed surgical implants—specifically, they’re bio‑absorbable fixation plates rather than temporary surgical tools.
Made from a resorbable polymer (polydioxanone), they are shaped and secured in place (for example, to reinforce septal cartilage or reconstruct orbital floors), provide structural support for several months, and then gradually hydrolyze and are absorbed by the body over the following 6–12 months
Resources
https://primismedical.com/products
https://fda.report/GUDID/20705031132532
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=15614460&pc=