Understand the differences between darkfield and brightfield microscopy. Learn about the types, advantages, and applications of each method. Discover key facts and insights into how these microscopy techniques are used in scientific research and diagnostics.
The difference between brightfield and darkfield microscopy is their illumination method, with brightfield using direct light through the specimen for a dark-on-light image and darkfield using light from the sides for a bright-on-dark image. Brightfield requires staining for contrast in transparent samples, whereas darkfield naturally enhances contrast without stains. While brightfield excels at detailing absorbing features, darkfield is superior for observing scattering features like specimen edges.
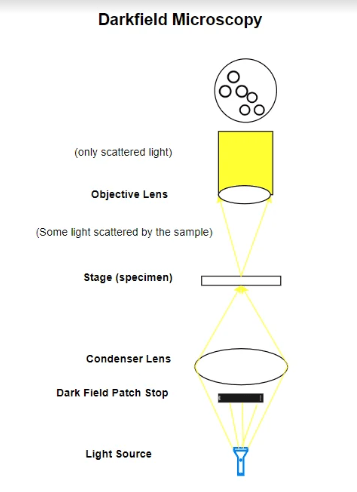
Darkfield microscopy is a technique that enhances the contrast in unstained, transparent specimens. It achieves this by directing light towards the specimen at an angle. Rather than illuminating the specimen directly, most of the light is either diffracted or misses the objective lens.
As a result, only the light scattered by the specimen enters the objective, making the specimen appear brightly lit against a dark background. This method is particularly useful for observing specimens that are invisible or hard to distinguish in standard brightfield microscopy, such as live bacteria, protozoa, and delicate tissues.
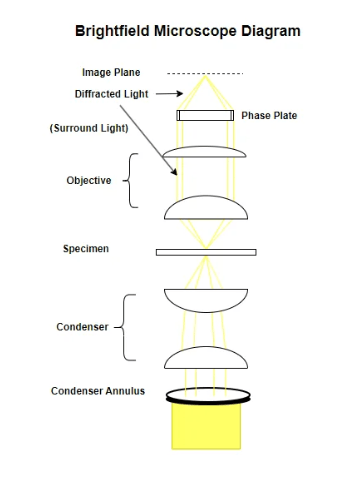
Brightfield microscopy is an optical microscopy technique that illuminates a specimen directly with bright light, causing the specimen to appear dark against a light background. A brightfield microscope is a tool designed for this technique, featuring a light source and lenses to magnify and visualize the specimen, usually used for observing stained biological samples to enhance contrast.
Brightfield microscopy is suitable for viewing fixed and live cells when combined with specific staining techniques to increase contrast, since unstained cells are often transparent and lack contrast in this setup. However, its utility is limited for visualizing fine details in unstained, transparent specimens due to inherently low contrast.
| Darkfield Microscopy | Brightfield Microscopy |
|---|---|
| Bright image on a dark background | Dark image on a bright background |
| No staining required | Requires staining for most specimens |
| Ideal for live specimens | Best for fixed, stained specimens |
| High contrast images | Low contrast images without staining |
| Uses scattered light | Uses transmitted light |
| Details external structures | Shows internal structures |
| Less common in basic labs | Standard in most labs |
| Requires special condensers (e.g., cardioid) | Uses simple Abbe condensers |
| Expensive setup | More affordable setup |
| Not suitable for thick specimens | Suitable for a variety of specimen thicknesses |
| Complex specimen preparation not required | Complex specimen preparation often required |
| Shows surface details and edges | Shows general morphology |
| Ideal for observing motility | Limited in observing non-stained motility |
| Harder to use for quantitative measurements | Easier for quantitative analysis |
| Can study minerals and metals | Not suitable for opaque materials |
| Difficult to photograph due to high brightness | Easier to photograph |
| Low light levels at specimen | High light levels at specimen |
| Can cause less photo-damage to live specimens | May cause photo-damage to sensitive specimens |
| Useful in detecting small particles | Less effective in detecting unpigmented particles |
| Requires more skilled operation | Simpler to operate for beginners |
Darkfield vs Brightfield Microscopy: Detailed Differences
Image Contrast, Background, and Specimen Requirements: Darkfield microscopy creates images with specimens brightly illuminated against a dark background, enhancing contrast for uncolored, transparent specimens without staining. This method is useful for observing live, unstained specimens, preserving their natural state and colors for real-time study.
In contrast, brightfield microscopy shows specimens as darker features on a bright background, requiring fixation and staining to improve contrast and visibility. This process can prevent observing specimens in their natural state and might alter or obscure details, making brightfield less suitable for studying live organisms and dynamic processes compared to darkfield microscopy.
Contrast Mechanism and Specimen Suitability: Darkfield microscopy utilizes light scattered by the specimen to highlight fine details and edges against a dark background, making it ideal for observing external structures and motility in live organisms like bacteria and protozoa.
Conversely, brightfield microscopy depends on light absorption and transmission through the specimen, offering less detailed contrast without staining but is versatile across a wide range of applications, including clinical diagnosis and basic biology, particularly effective for internal structures when specimens are properly stained.
Technical, Cost, Photography, and Analysis Considerations: Darkfield microscopy, requiring special condensers and higher initial investment, is less accessible than brightfield setups, straightforward and cost-effective for beginners and educational settings. Capturing darkfield images poses challenges with brightness and contrast, needing adjustments and experience, whereas brightfield provides easier photography and analysis for quantitative studies with clearer specimen definition.
Specimen Thickness, Preparation, and Material Study: Darkfield microscopy struggles with thick specimens due to light scattering issues, while brightfield microscopy, suitable for various thicknesses, needs complex preparation like sectioning and staining. Darkfield excels in studying opaque materials like minerals and metals by capturing reflected light, unlike brightfield, which requires light transmission through the specimen.
Light Levels, Photodamage, and Detecting Small Particles: Darkfield microscopy’s lower light levels reduce photodamage risk, safeguarding delicate, live specimens, while its high-contrast illumination excels at detecting small particles and subtle details crucial in fields like microbiology and hematology. Conversely, brightfield microscopy’s higher light levels can cause photodamage, especially in light-sensitive specimens.
Darkfield Microscopy

Shigella dysenteriae bacteria revealed through dark field microscopy.
Uses of Darkfield Microscopy
- Medical Diagnosis and Research: Darkfield microscopy is vital for visualizing hard-to-see microorganisms such as spirochetes, including those responsible for syphilis (Treponema pallidum), Lyme disease (Borrelia burgdorferi), and leptospirosis (Leptospira interrogans). It’s also crucial for observing microbial motility and studying non-culturable bacteria in patient samples.
- Biological Studies: Utilizing darkfield microscopy allows for the detailed observation of the internal structures of larger microorganisms like algae and yeasts, as well as the study of microbial motility. This technique is also applied to view blood cells, providing insights into their morphology and behavior.
- Environmental and Marine Biology: This technique is invaluable for studying various environmental samples, including different types of algae and marine organisms such as plankton and diatoms, enhancing our understanding of these ecosystems.
- Material Science and Metallurgy: Darkfield microscopy serves an important role in examining material properties, including the observation of hairline metal fractures. This aids in the analysis of materials’ structural integrity and the investigation of failures.
- Forensic and Scientific Investigation: In forensic science, darkfield microscopy is used to examine fibers, hairs, and other small pieces of evidence. This technique provides detailed visual information that can be crucial in solving crimes or understanding complex scientific phenomena.
Darkfield Microscope Diagram
Pros and Cons of Darkfield Microscopy
Pros of Darkfield Microscopy:
- Improved Image Contrast: Darkfield microscopy improves image contrast without the need to stain samples. This is beneficial for professions such as microbiologists and biologists who need to observe living cells without killing them.
- Resolution: The resolution by dark-field microscopy is somewhat better than bright-field microscopy. This can be advantageous for researchers and scientists studying detailed structures within cells.
- Direct Detection of Non-culturable Bacteria: Darkfield microscopy allows for the direct detection of non-culturable bacteria present in patient samples. This is particularly useful for medical professionals and clinical microbiologists when diagnosing diseases.
- No Sample Preparation Required: Darkfield microscopy requires no special sample preparation. This can save time and resources for lab technicians and researchers.
- Visualization of Transparent Specimens: Darkfield microscopy is most useful when researchers need to visualize unstained, transparent specimens. This makes it a valuable tool for professions such as marine biologists studying aquatic organisms.
Cons of Darkfield Microscopy:
- Limited to Wet, Moist Specimens: Darkfield microscopy requires the examination of wet, moist specimens containing living organisms very quickly. This can be a limitation for field researchers who may not have immediate access to a microscope.
- Strong Illumination Required: The sample must be very strongly illuminated, which can cause damage to the sample. This could be a disadvantage for researchers studying sensitive or delicate specimens.
- Dust Particles Appear Bright: Besides the sample, dust particles also scatter the light and appear bright. This can lead to inaccurate results, affecting professions such as forensic scientists who require precise observations.
- Sample Material Needs to be Spread Thinly: Dense preparations can grossly affect the contrast and accuracy of the dark field’s image. This could be a limitation for histologists who often work with thicker tissue sections.
- Prone to Degradation, Distortion, and Inaccuracies: Dark field images are prone to degradation, distortion, and inaccuracies. This can be a disadvantage for professions such as pathologists who rely on accurate images for diagnosis.
Types of Darkfield Microscopy
- Biological Darkfield Microscope: Primarily utilized in biological research for observing live, unstained samples, such as algae, this microscope employs a special condenser to direct light away from the lens, creating a contrast that highlights organisms against a dark background. Oil immersion and a specific numerical aperture (NA) are employed to enhance the visibility of minute details without staining the specimens.
- Metallurgical Darkfield Microscope: Specialized for inspecting metal structures, this microscope reveals hairline fractures and imperfections in metals through an annular aperture that focuses light onto the sample, producing a detailed view of the material’s surface and subsurface structures. It is equipped with features such as a quintuple nosepiece, Plan objectives, and the capability for EPI and transmitted illumination, making it ideal for detailed analysis in materials science.
- Gemological Microscope (Stereo Darkfield Microscope for Gems): Designed for the gemology field, this microscope is equipped with strong zoom, high levels of magnification, and darkfield illumination to illuminate diamonds and precious stones from below, enhancing visibility of inclusions and imperfections. Additional functionalities often include camera or video capabilities, as well as accessories like tweezers or clamps for securely holding and manipulating gems during examination.
- Stereo Darkfield Microscope for Biological Samples: Utilized for observing larger specimens, such as shrimp and invertebrates, this type employs an inverted cone of light directed at oblique angles by a specialized stand with a reflection mirror and light-shielding plate. This setup, combining the principles of darkfield illumination with stereo microscopy, allows detailed three-dimensional viewing of specimens, making it perfect for applications in biology and ecology that require examination of the structure and behavior of organisms in their natural, unaltered state.
Facts About Darkfield Microscopy
- Darkfield microscopy was first developed in the 1840s.
- The technique was initially used to resolve the striae of a diatom species known as Navicula Spencerii.
- Charles Spencer, a New York lens maker, was the person after whom this diatom was named.
- The use of oblique light was found to be necessary to resolve the striae, marking the first step towards darkfield microscopy.
- Darkfield microscopy became popular due to its use in the discovery of Treponema pallidum, the syphilis spirochete, by Schaudinn in 1905.
- The discovery promoted the use of darkfield microscopy by the medical profession and subsequently by biologists in general.
- Darkfield microscopy is the only wide field method that does not strictly use Köhler illumination.
- In darkfield microscopy, no direct light from the condenser enters the objective lens.
- The first condenser made specifically for darkfield was produced by Francis H. Wenham and George Shadbolt in 1855.
- This condenser used a parabolic glass reflector to create a hollow cone of light.
Brightfield Microscopy
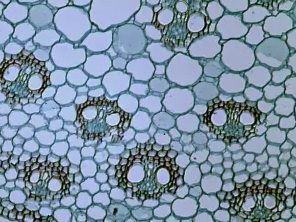
This is a 100× magnified, bright-field micrograph image showing a cross-section of a Zea stem.
John Alan Elson, CC BY-SA 4.0, via Wikimedia CommonsUses of Brightfield Microscopy
- Biological Research and Medical Applications: Brightfield microscopy is essential for examining the microcosmos of life, including the detailed study of animal and plant cells, bacterial morphology, and the identification of protozoans. It also plays a crucial role in medical research by providing detailed views of biological samples, aiding in disease diagnosis and furthering our understanding of life at the microscopic level.
- Microbiological Exploration: In microbiology labs, brightfield microscopy serves as the cornerstone for observing both fixed and live specimens. Whether for educational purposes or advanced research, it allows scientists to delve into the microscopic world, studying microorganisms and their behaviors.
- Geological and Material Science Investigations: Beyond biological applications, brightfield microscopy is employed in studying the earth’s components and various materials. Geologists use it to examine rock and earth samples for hidden features, while material scientists explore the microstructures of minerals, metals, and other non-biological materials.
- Educational Tool: Its simplicity, affordability, and effectiveness make brightfield microscopy a preferred tool in educational settings. From schools to universities, it facilitates science education by allowing students to observe and understand the microscopic world firsthand.
- Industrial and Quality Control: Brightfield microscopy is not confined to scientific research; it extends into industrial applications, serving as a general imaging technique for specimen observation and quality control across various sectors. This underscores its adaptability and utility in both scientific and practical contexts.
Brightfield Microscope Diagram
Pros and Cons of Brightfield Microscopy
Pros of Brightfield Microscopy:
- Simple to Use: Brightfield microscopy is user-friendly, making it a great tool for beginners or those needing quick observations. This makes it ideal for educational settings where students are just beginning to learn about microscopy.
- Low-Cost Equipment: Compared to advanced microscopy techniques, brightfield microscopes are more affordable. This makes them accessible for schools and small labs operating on a budget.
- No Staining Required for Some Specimens: Certain specimens like bacteria can be seen without adding dyes. This saves time and preserves the natural state of the specimen, which is beneficial for microbiologists and bacteriologists.
- Clear Images of Live Samples: Brightfield microscopy allows for the study of behaviors and life processes as they happen. This is particularly useful for biologists studying cell dynamics.
- Widely Available Technology: This method is common and easy to find in most educational and research settings. This ensures that many people can use it without having to search for specialized equipment.
Cons of Brightfield Microscopy:
- Low Contrast for Transparent Specimens: Transparent samples often appear almost invisible under brightfield microscopy because there’s not much difference in light absorption between the specimen and the surrounding medium. This can be a challenge for researchers studying transparent organisms or cells.
- Limited Resolution: The technique doesn’t provide the finest detail because it’s limited by the wavelength of light. This makes it less suitable for researchers who need to see really tiny structures.
- No Depth Perception: It gives a flat image, making it tricky to tell the position of structures in layered or thick samples. This can be a limitation for histologists studying tissue sections.
- Cannot View Living Cells Well: Observing live cells is difficult as they’re usually transparent and move around, plus the light can damage or kill them. This is a drawback for cell biologists studying live cell interactions.
- Poor for Thick Specimens: It’s not great for looking at thick samples because the light doesn’t penetrate deeply. So parts of the specimen can look blurry or be missed entirely. This can be a problem for researchers studying larger or denser specimens.
Types of Brightfield Microscopy
- Brightfield Microscope (Compound Light Microscope): An optical instrument designed for seeing dark images against a bright background using light illumination. It’s widely used in biological, cellular biology, and microbiological studies. Comprising parts such as eyepieces, objective lenses, and focusing knobs, it’s suitable for observing stained specimens that contrast sharply with their background via light absorption and refraction.
- Phase Contrast Microscopy: Enhances contrast in unstained samples without damaging them, ideal for live cell observation. It operates by amplifying phase shifts in light passing through transparent specimens, thus creating clear, detailed images of cells and their components, making it crucial for studying biological processes.
- Differential Interference Contrast (DIC) Microscopy: Produces pseudo-three-dimensional images by using polarized light that generates contrast in specimens with varying thickness or refractive indices. Particularly useful for visualizing features like organelles within cells and providing detailed views of live, unstained specimens.
- Polarized Light Microscopy: Employs polarized light to increase contrast in samples, especially those with birefringent materials such as crystals or biological structures like muscle fibers and cell walls. This technique is invaluable for studies requiring detailed analysis of material properties or structural characteristics within a sample.
Facts About Brightfield Microscopy

- Brightfield microscopy has been one of the most widely used observation modes in optical microscopy for the past 300 years.
- The brightfield microscope is an example of a compound microscope, meaning light from the object you are viewing passes through two lenses before it reaches your eye.
- Brightfield microscopy is best suited for utilization with fixed, stained specimens or other kinds of samples that naturally absorb significant amounts of visible light.
- Images produced with brightfield illumination appear dark and/or highly colored against a bright, often light gray or white, background.
- The brightfield microscope provides increased resolution, which is the ability to distinguish two points as separate points.
- A light microscope, such as a brightfield microscope, can improve resolution by as much as 1000-fold.
- Discernment of cellular detail can be improved with the use of dyes that add color and contrast to subcellular structures in brightfield microscopy.
- In the history of microscopy, brightfield microscopy goes back 400 years, to when European scientists first discovered a way to see cells using magnifying lenses, a metal tube, and a light source.
- In the modern era, quantitative bright-field absorbance microscopy (QBAM) combined with deep neural networks (DNNs) has been developed to non-invasively predict tissue function and cellular donor identity.
- The AbsorbanceQ app converts brightfield microscope images into absorbance images that can be analyzed and compared across different operators, microscopes, and time.
How does Darkfield and Brightfield Microsopy Compare to other Types of Microsopy?
- Phase Contrast Microscopy: Unlike brightfield microscopy, which relies on differences in light absorbance, phase contrast enhances contrasts in samples that are mostly transparent by converting phase differences in light passing through the specimen into variations in intensity. Darkfield microscopy illuminates the sample with scattered light, while phase contrast provides detailed internal structures without darkening the background.
- Differential Interference Contrast (DIC) Microscopy: DIC produces high-contrast images with a pseudo-3D appearance by utilizing polarized light, which neither darkfield nor brightfield microscopy can achieve. DIC highlights subtle differences in refractive indices, providing detailed textural information that darkfield and brightfield cannot.
- Fluorescence Microscopy: This technique involves staining the specimen with fluorescent dyes and illuminating it with specific wavelengths of light, causing the tagged components to emit longer wavelengths. Brightfield and darkfield microscopy don’t involve fluorescence; they rely on absorption (brightfield) or scattering (darkfield) of transmitted light, limiting their ability to specifically highlight individual components within cells.
- Confocal Microscopy: Offers higher resolution and contrast through a pinhole that eliminates out-of-focus light, unlike darkfield and brightfield microscopy that cannot selectively focus on different planes within a sample. Confocal microscopy enables 3D reconstruction of specimens, which is not feasible with traditional microscopy.
- Two-Photon (or Multiphoton) Microscopy: Uses longer-wavelength (near-infrared) light for excitation, reducing photodamage and allowing deeper tissue penetration than possible with darkfield or brightfield. This technique, involving simultaneous absorption of two photons, contrasts with the single-photon absorption in conventional microscopy.
- Scanning Electron Microscopy (SEM): Provides high-resolution, 3D images by bouncing electrons off the surface of a sample, a fundamentally different approach from darkfield and brightfield, which are limited by light diffraction and offer less depth perception.
- Transmission Electron Microscopy (TEM): Offers significantly higher resolution than darkfield and brightfield by transmitting electrons through the specimen. While darkfield and brightfield microscopy examine changes in light transmission or scattering, TEM visualizes the specimen’s inner structure based on electron transmission.
- Atomic Force Microscopy (AFM): Unlike both brightfield and darkfield, which rely on light, AFM uses a physical probe to scan the surface at the atomic level, enabling imaging of surfaces that are transparent or too thick for light microscopy.
- Super-Resolution Microscopy: Techniques like STED, PALM, and STORM surpass the resolution limits of traditional light microscopy (including darkfield and brightfield), allowing visualization of molecular processes within cells at unprecedented detail.
- Polarization Microscopy: Enhances contrast in birefringent materials by using polarized light, a contrast mechanism not available in conventional darkfield or brightfield microscopy, which don’t differentiate based on polarization.
- Total Internal Reflection Fluorescence (TIRF) Microscopy: Targets a thin region of the specimen near the glass slide, unlike darkfield and brightfield which illuminate the entire specimen. TIRF is excellent for observing surface phenomena, such as cell adhesion.
- Raman Microscopy: Offers chemical and structural information based on Raman scattering, a capability beyond the reach of darkfield and brightfield microscopy, which cannot provide molecular information about the specimen.
- Near-field Scanning Optical Microscopy (NSOM or SNOM): Achieves higher resolution than conventional optical methods by bypassing the diffraction limit, a significant advantage over darkfield and brightfield, which are constrained by optical wavelengths.
Resources
https://www.olympus-lifescience.com/en/microscope-resource/primer/techniques/darkfield/
This page offers an in-depth look at darkfield microscopy, a technique enhancing visibility of unstained, transparent specimens against a dark background by allowing only oblique rays to strike the specimen.
This page covers techniques in brightfield microscopy including phase contrast, darkfield, DIC, and polarized light to enhance contrast and details of transparent samples, explaining principles, applications, and providing example images.
https://www.azooptics.com/Article.aspx?ArticleID=2125
This page goes into the history and operation of dark-field microscopes, illuminating how they brightly light unstained cells and microorganisms against a dark background for high-contrast observation and highlighting their evolution.
This page provides a brief history of light microscopy from its origins in the Middle Ages using simple magnifying lenses to modern developments like super-resolution imaging and confocal microscopy.









