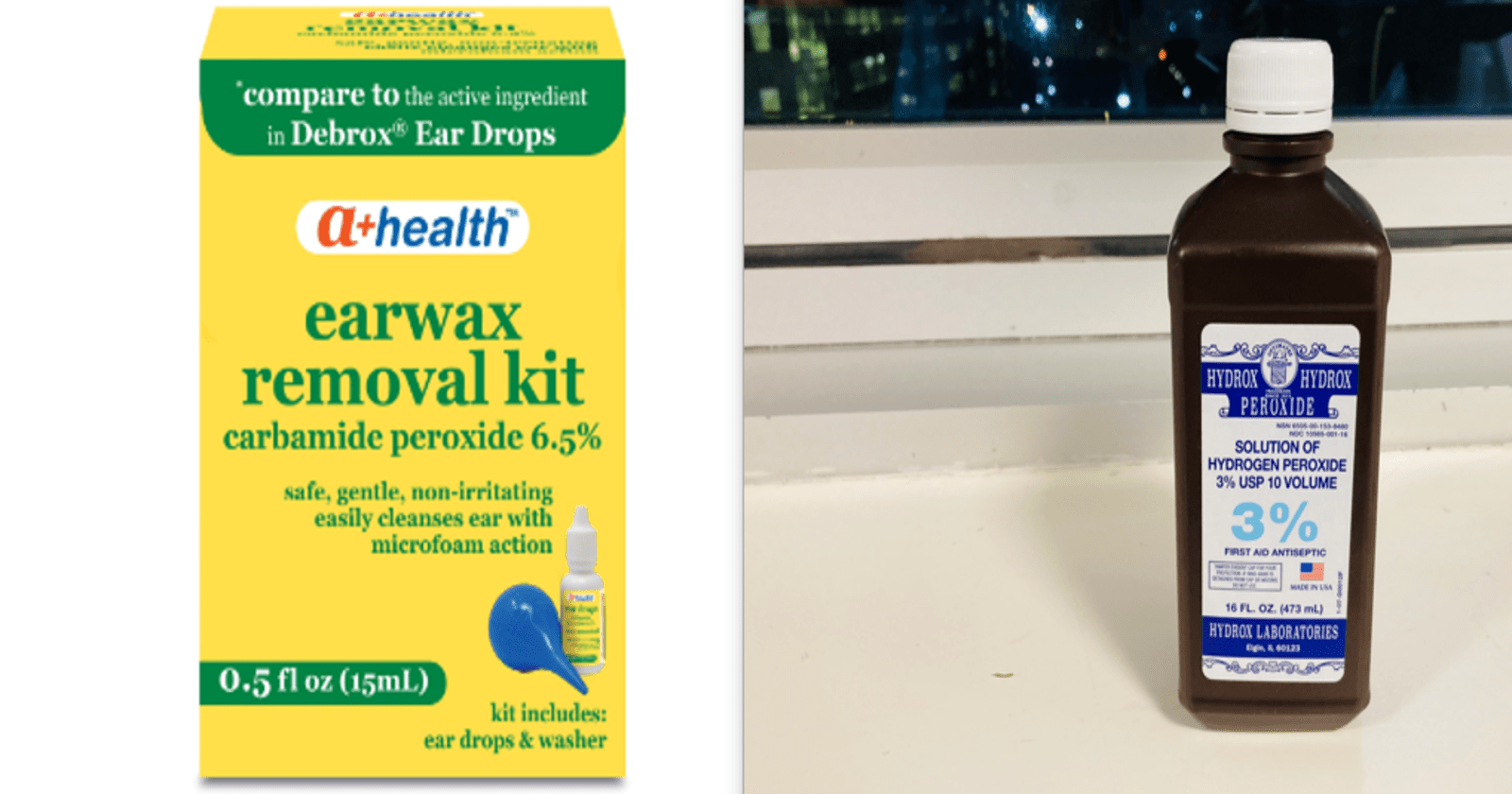
Carbamide peroxide is a water-soluble, white crystalline solid compound consisting of hydrogen peroxide and urea, source of hydrogen peroxide, it can be found in disinfecting and dental bleaching products.
Hydrogen peroxide (H₂O₂) is a clear liquid made of hydrogen and oxygen. It’s commonly used as a disinfectant to clean wounds and surfaces because it kills bacteria and viruses. You can also find it in hair bleaching products and as a teeth whitening agent.
Pure hydrogen peroxide is a slightly thicker, very light blue liquid. It’s primarily used as an oxidizer, bleaching agent, and antiseptic. For everyday use, it’s diluted in water, while industries use higher concentrations.
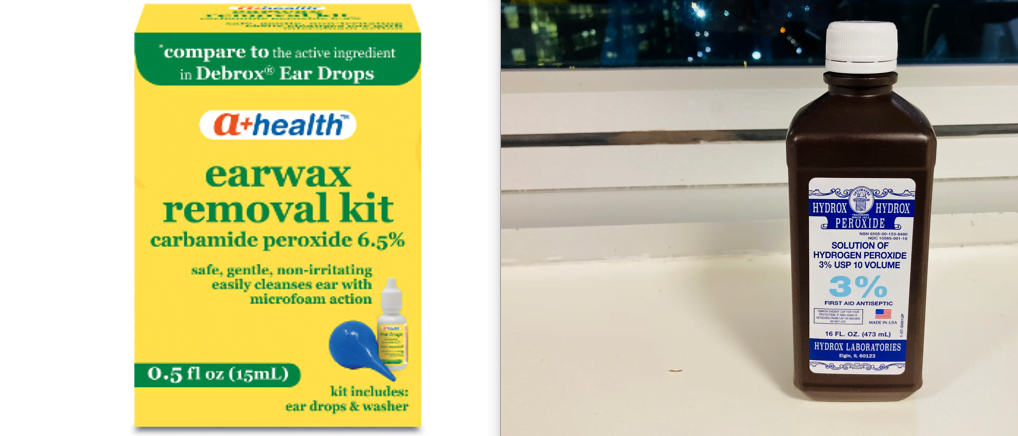
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| Contains hydrogen and urea | Contains hydrogen and oxygen |
| Typically lower strength concentration | Typically higher strength concentration |
| Relatively stable and dose not decompose quickly | Less stable, decomposes quickly |
| Often used in dental products | Used as a disinfectant, bleach |
| Penetrates enamel more slowly | Penetrates enamel more quickly |
| Bleaching action is Gradual, over longer periods | Bleaching action is Faster, more immediate effect |
| Less likely to cause sensitivity | More likely to cause sensitivity |
Health benefits of Carbamide peroxide
1. Teeth Whitening: Carbamide peroxide breaks down into hydrogen peroxide, which acts as a bleaching agent. When applied to teeth, it can help remove stains and lighten the color of teeth, leading to a brighter smile.
2. Dental Hygiene: Carbamide peroxide has antimicrobial properties, helping to kill harmful bacteria in the mouth. This can contribute to improved oral hygiene and reduced risk of gum disease and cavities.
3. Treating Mouth Ulcers: Some dental professionals use carbamide peroxide to help heal mouth ulcers. Its antiseptic properties can reduce inflammation and promote healing when applied topically to ulcers.
4. Ear Wax Removal: Carbamide peroxide can be used to soften and loosen earwax, making it easier to remove. It’s often found in over-the-counter earwax removal kits and can help prevent blockages and discomfort.
5. Wound Cleansing: In diluted form, carbamide peroxide can be used to clean minor wounds and cuts. Its antiseptic properties help kill bacteria and prevent infection, promoting faster healing.
Health benefits of Hydrogen peroxide
1. Antiseptic Properties: Hydrogen peroxide is an effective antiseptic agent, killing bacteria, viruses, and fungi. It’s commonly used to clean and disinfect wounds, cuts, and scrapes, reducing the risk of infection.
2. Oral Health: Diluted hydrogen peroxide can be used as a mouthwash to kill harmful bacteria in the mouth, promote gum health, and freshen breath. It’s also found in some toothpaste formulations for its whitening properties.
3. Ear Care: Hydrogen peroxide is used to soften and remove excess earwax buildup, helping to prevent blockages and reduce the risk of ear infections. It’s often used in combination with warm water to gently flush out the ear canal.
4. Skin Care: Hydrogen peroxide can be applied topically to treat acne, minor skin infections, and fungal conditions like athlete’s foot. Its antiseptic properties help clean the affected area and promote healing.
5. Dental Whitening: Hydrogen peroxide is a key ingredient in many teeth whitening products. It’s used to remove surface stains and lighten the color of teeth, resulting in a brighter smile.

6. Sinus Infections: Some people use diluted hydrogen peroxide as a nasal spray to help alleviate symptoms of sinus infections and nasal congestion. It may help clear nasal passages and reduce inflammation.
7. Household Disinfectant: Hydrogen peroxide can be diluted and used as a non-toxic household cleaner and disinfectant. It’s effective at killing bacteria and viruses on surfaces without leaving harmful residues.
While hydrogen peroxide has many potential health benefits, it’s essential to use it properly and in appropriate concentrations. Concentrated hydrogen peroxide can cause skin irritation, burns, and other adverse effects if not diluted correctly. It’s also crucial to consult with a healthcare professional before using hydrogen peroxide for any medical purposes, especially if you have underlying health conditions or concerns.
Uses of Carbamide peroxide vs Hydrogen peroxide
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| Teeth Whitening | Antiseptic for Wound Cleaning |
| Dental Hygiene | Oral Health (as mouthwash and teeth whitener) |
| Treating Mouth Ulcers | Ear Wax Removal |
| Ear Wax Removal | Skin Care (acne treatment, wound disinfection) |
| Wound Cleansing | Dental Whitening |
| Treat mouth ulcer | Sinus Infections (nasal spray) |
| Household Disinfectant (when diluted) |
Both Carbamide peroxide and Hydrogen peroxide have overlapping uses in dental care, wound cleaning, and oral health, but they may differ in concentration and application. While Carbamide peroxide is primarily used for dental purposes like teeth whitening and mouth ulcer treatment, Hydrogen peroxide has a broader range of applications including wound cleansing, ear care, skin care, and household disinfection.
As of January 08, 2024 , U.S. Food and Drug Administration (FDA ) state that Vaporized hydrogen peroxide (VHP) is to be a method of sterilization for medical devices.
Advantages of Carbamide peroxide vs Hydrogen peroxide
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| More stable, longer shelf life | Less stable, shorter shelf life |
| Releases oxygen more gradually | Releases oxygen quickly |
| Generally less irritating to gums | Can be more irritating to gums |
| May provide longer-lasting whitening | May provide quicker results, but shorter-lasting |
| Typically lower concentration, safer | Higher concentration, may require more caution |
| Usually more cost-effective | Can be more expensive |
Disadvantages of carbamide peroxide vs Hydrogen peroxide
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| Typically requires longer treatment times | Generally provides quicker results |
| Less potent, may require more applications | More potent, potential for enamel damage if used incorrectly |
| May cause tooth sensitivity in some individuals | More likely to cause tooth sensitivity |
| Can have a stronger and less pleasant taste | Generally has a milder taste |
| Less invasive due to slower release of oxygen | More invasive due to rapid release of oxygen |
How Carbamide peroxide works in teeth whitening and earwax removal
Carbamide peroxide is a widely used compound known for its applications in teeth whitening and earwax removal. Its effectiveness can indeed vary based on several factors, including concentration, pH, and formulation.

1. Concentration: The concentration of carbamide peroxide in a product significantly affects its effectiveness. In teeth whitening products, higher concentrations are typically used for faster results, while lower concentrations are used for longer treatments with less risk of sensitivity. Higher concentrations can lead to faster whitening but may also increase the risk of tooth sensitivity and soft tissue irritation. Conversely, lower concentrations may require more extended treatment periods but are generally gentler on the teeth and gums.
2. pH: pH plays a crucial role in determining the stability and activity of carbamide peroxide. Carbamide peroxide is most effective in a slightly acidic environment. pH influences the rate of decomposition of carbamide peroxide into hydrogen peroxide and urea, which are the active components responsible for whitening and softening earwax, respectively. An optimal pH ensures the stability of the compound and enhances its efficacy. For example, teeth whitening gels often contain buffering agents to maintain the pH within an optimal range for maximum effectiveness.
3. Formulation: The formulation of carbamide peroxide products can vary significantly depending on the intended application. For teeth whitening, carbamide peroxide is commonly found in gels, strips, or trays, which allow for direct application to the teeth. These formulations often contain additional ingredients such as desensitizing agents to minimize tooth sensitivity and flavoring agents for better user experience. In contrast, for earwax removal, carbamide peroxide is usually found in the form of ear drops, which are designed to soften and facilitate the removal of earwax buildup.
How Hydrogen peroxide works in teeth whitening and earwax removal
1. Concentration: The concentration of hydrogen peroxide greatly affects its efficacy. Higher concentrations typically have stronger antimicrobial properties due to their ability to release more oxygen radicals, which can damage bacterial cell membranes and DNA. However, higher concentrations can also be more corrosive and irritating to tissues. Lower concentrations are safer for use on sensitive tissues but may require longer exposure times or repeated applications to achieve the desired effect.
- Teeth Whitening: In teeth whitening products, hydrogen peroxide is often used in concentrations ranging from 3% to 35%. Higher concentrations are typically used in professional treatments for quicker results, but they may increase the risk of tooth sensitivity and gum irritation.

- Earwax Removal: For earwax removal, concentrations around 3% are commonly used. This concentration is effective at softening and loosening earwax without causing irritation to the delicate tissues of the ear canal
2. pH: The pH level of a hydrogen peroxide solution also influences its effectiveness. Hydrogen peroxide is most stable and effective at acidic to neutral pH levels. At lower pH values, it tends to decompose more slowly, releasing oxygen more gradually. Extreme pH values can destabilize hydrogen peroxide and reduce its effectiveness.
- Teeth Whitening: Teeth whitening products often contain buffering agents to maintain the pH within an optimal range for hydrogen peroxide activity. The acidic environment helps to enhance the whitening process by facilitating the breakdown of stains on the teeth.
- Earwax Removal: Solutions used for earwax removal are typically close to neutral pH to minimize irritation to the delicate skin of the ear canal.
3. Formulation: The formulation of hydrogen peroxide products can also impact their efficacy. Factors such as the presence of stabilizers, surfactants, and other additives can affect the stability, penetration, and overall performance of the product.
- Teeth Whitening: Teeth whitening gels or strips often contain hydrogen peroxide along with other ingredients such as carbamide peroxide, which breaks down into hydrogen peroxide and urea. These formulations may also include desensitizing agents to minimize tooth sensitivity.
- Earwax Removal: Ear drops for earwax removal may contain hydrogen peroxide in combination with other ingredients such as glycerin or olive oil to help soften and disperse the wax.
How dose Carbamide peroxide and Hydrogen peroxide REACTS when use together
Carbamide peroxide breaks down into urea and hydrogen peroxide when applied to the teeth or ear canal. When used together, hydrogen peroxide may enhance the whitening effect of carbamide peroxide.
When combined, carbamide peroxide and hydrogen peroxide work synergistically to break down stains on teeth or surfaces. The hydrogen peroxide released from the breakdown of carbamide peroxide acts as the active bleaching agent, penetrating the enamel and breaking down pigmented molecules that cause discoloration. This process effectively whitens teeth or cleans surfaces.
The reaction between carbamide peroxide and hydrogen peroxide is typically rapid, especially in the presence of moisture. However, the exact reaction kinetics and mechanisms can vary depending on the concentration of the solutions, pH levels, and other factors. Overall, their combination is often used in various dental and cleaning products to achieve optimal results in whitening or disinfection.
Which is better hydrogen peroxide or carbamide peroxide?
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| Teeth Whitening, it effectively oxidizes and removes stains from the surface of teeth | Wound Cleaning used to clean minor cuts, scrapes reducing the risk of infection. |
| Gradual Whitening, best for individual with tooth sensitivity | Surface Disinfection, used to sanitize countertops, cutting boards, and other surfaces. |
| Lower Sensitivity, best for individuals with allergies and high sensitivity to hydrogen peroxide | Oral Care-antiseptic properties kills bacteria in the mouth |
| Longer Shelf Life-remains effective for a longer period. | Laundry Whitening-added to laundry to help whiten whites and remove stains. |
| Customized Trays-distribute whitening gel tailored to fit the individual’s teeth, maximizing effectiveness and minimizing gum irritation. | Hair Bleaching-lighten their hair or create highlights. It can be applied directly to the hair to break down the pigments and achieve a lighter color. |
| At-Home Whitening-accessible and convenient way to whiten their teeth at home. | Mouth/Gum Infections-help alleviate symptoms of mouth and gum infections, such as gingivitis. |
Hydrogen peroxide-acts as a bleaching agent and is particularly useful for treating stains caused by blood, sweat, or food.
After a bloody trauma and surgery in the operating room we usually soak the floor with hydrogen peroxide and leave for a few minutes, to remove all blood stain and disinfects.
Warning when Using Carbamide peroxide and Hydrogen peroxide
Carbamide peroxide
1. Avoid Ingestion: Carbamide peroxide products, such as teeth whitening gels or ear wax removal solutions, are intended for external use only. Ingestion of these products can lead to irritation of the digestive tract, nausea, vomiting, and potentially more severe health effects. Keep them out of reach of children and pets.

2. Potential Irritation: Carbamide peroxide can cause irritation to oral tissues, gums, or the ear canal if used improperly or in excessive amounts. Follow the instructions provided with the product carefully, and discontinue use if you experience any discomfort or irritation.
3. Sensitivity: Some individuals may experience increased tooth sensitivity or gum irritation when using teeth whitening products containing carbamide peroxide. If you have a history of dental sensitivity or gum problems, consult with your dentist before starting a whitening regimen.
4. Avoid Contact with Eyes: When applying carbamide peroxide solutions to the ear canal or using whitening trays for teeth, avoid contact with the eyes. Rinse thoroughly with water if accidental contact occurs and seek medical attention if irritation persists.

5. Pregnant or breastfeeding women, individuals with untreated dental problems, or those with a history of allergic reactions to peroxide-based products, may not be suitable candidates for carbamide peroxide treatments. Consult with a healthcare professional before using these products if you have any concerns.
6. Household Chemicals: Mixing carbamide peroxide with certain household chemicals can result in chemical reactions or produce harmful byproducts. Avoid combining it with acidic substances or bleach-based cleaners, as this can lead to the release of toxic gases or cause skin and eye irritation. It’s essential to follow product instructions and use carbamide peroxide in a well-ventilated area.
Hydrogen peroxide
1. Avoid Ingestion: Hydrogen peroxide is a strong oxidizing agent and can cause harm if ingested. It can lead to irritation of the gastrointestinal tract, abdominal pain, vomiting, and in severe cases, tissue damage or perforation of the stomach. Store hydrogen peroxide solutions securely and out of reach of children and pets.

2. Irritation and Sensitivity: Hydrogen peroxide can cause irritation or sensitivity when applied to the skin, mucous membranes, or oral tissues. Avoid contact with the eyes, mouth, and sensitive areas of the skin. Rinse thoroughly with water if contact occurs and seek medical attention if irritation persists.
3. Do Not Inhale: Inhalation of hydrogen peroxide vapors can irritate the respiratory tract and cause coughing, shortness of breath, or chest tightness. Use hydrogen peroxide solutions in a well-ventilated area and avoid breathing in the vapors directly.
4. Dilution: Concentrated hydrogen peroxide solutions should be diluted before use, especially for topical applications or wound cleansing. Follow the recommended dilution ratios provided by the manufacturer or healthcare professional to ensure safe and effective use.
5. Not for Internal Use: Hydrogen peroxide solutions intended for external use only should not be ingested or used internally, unless specifically directed by a healthcare professional. Ingestion of undiluted hydrogen peroxide can lead to serious health complications, including chemical burns and systemic toxicity.
6. Avoid Mixing with Certain Substances: Mixing hydrogen peroxide with certain substances, such as acids or organic materials like acetone, can result in chemical reactions that release heat, oxygen, or toxic gases. These reactions can be hazardous and may cause fire, explosions, or the formation of harmful byproducts. Always use hydrogen peroxide with caution and avoid mixing it with incompatible materials.
How to Store Carbamide peroxide and Hydrogen peroxide
Storing Carbamide peroxide
Storage: Store carbamide peroxide products in a cool, dry place, away from direct sunlight and heat sources. Keep the containers tightly closed to prevent evaporation or contamination. Check the expiration date and discard any expired or deteriorated products.
Storing Hydrogen peroxide
Storage and Handling: Store hydrogen peroxide solutions in a cool, dry place, away from direct sunlight, heat sources, and incompatible materials. Keep the containers tightly closed to prevent evaporation and contamination. Check the expiration date and discard any expired or deteriorated products.
Recommended time for using 10% and 15% hydrogen peroxide for teeth whitening:
| Hydrogen Peroxide Concentration | Recommended Duration |
|---|---|
| 10% how long on teeth | 30-60 minutes |
| 15% how long on teeth | 15-20 minutes |
Side effects of Carbamide peroxide and Hydrogen peroxide
Generally considered safe when used as directed, Both Carbamide peroxide and hydrogen peroxide can cause some side effects.
| Carbamide Peroxide | Hydrogen Peroxide |
|---|---|
| hives | Tooth Sensitivity |
| swollen face, lips, tongue, or throat, | Gum Irritation |
| Temporary whitening of gums and oral tissues | Oral Mucosa Irritation |
| Ear irritation (for earwax removal products) | Temporary Whitening of Gums and Oral Tissues |
| Tastes and Odors | Tastes and Odors |
| Rare allergic reactions | Ear Irritation (for earwax removal products) |
| Allergic Reactions |
Environmental Impact of carbamide peroxide
1. Breakdown Products: Carbamide peroxide typically breaks down into urea and hydrogen peroxide. Urea is a natural compound found in urine and is generally not considered harmful to the environment in moderate concentrations. Hydrogen peroxide, however, can be both beneficial and harmful. In low concentrations, it can degrade into water and oxygen, making it relatively harmless. However, in higher concentrations, it can act as a potent oxidizing agent, potentially causing harm to aquatic organisms.
2. Persistence: Carbamide peroxide is not considered highly persistent in the environment. Its breakdown products, urea and hydrogen peroxide, are relatively short-lived and tend to degrade relatively quickly under normal environmental conditions. However, in certain environments with limited oxygen or microbial activity, such as sediments or groundwater, they may persist for longer periods.
3. Toxicity to Non-target Organisms: While urea is generally considered non-toxic to most organisms at typical environmental concentrations, hydrogen peroxide can be toxic, especially to aquatic organisms such as fish and invertebrates. The extent of toxicity depends on factors such as concentration, exposure duration, and the sensitivity of the species involved. Studies have shown that high concentrations of hydrogen peroxide can lead to adverse effects on aquatic ecosystems, including fish mortality and disruption of aquatic food chains.
4. Research and Concerns: Research on the environmental impact of carbamide peroxide is ongoing, with a focus on understanding its fate and behavior in different environmental compartments, such as water, soil, and sediment. Concerns regarding its potential environmental effects stem from its widespread use in various consumer products, including tooth whitening agents, mouthwashes, and hair dyes, which can lead to its release into wastewater systems and subsequently into the environment.
Research Assessment of 16% and 35% carbamide peroxide for professional in-office teeth whitening.
| Aspect | Findings |
|---|---|
| Participants | Thirty adult subjects (20 females, 10 males) aged 18-43 years participated (mean age 27.83 years) |
| Bleaching Agents | Carbamide peroxide in concentrations of 16% and 35% |
| Technique | Split-arch technique/half-mouth design using vacuum formed half-arch matrix seated over the maxillary teeth |
| Duration | One-hour session per week for two weeks |
| Outcome Assessment | Vita shade guide and photographs |
| Results | Tooth whitening achieved in all subjects |
| Shade tab movement recorded: – 16% concentration: 2-4 shades (first session), 3-5 shades (second session) – 35% concentration: 4-6 shades (first session), 5-9 shades (second session) | |
| Comparison of Bleaching Outcome | Significantly better outcome with 35% concentration of carbamide peroxide |
| Side Effects | Transient tooth sensitivity observed with both concentrations of the bleaching agents |
| Conclusion | Both 16% and 35% concentrations of carbamide peroxide are effective and safe for bleaching discolored vital teeth. – 35% concentration provided significantly greater lightening effect without additional side effects compared to 16% concentration. |
Resources
https://www.medicinenet.com/carbamide_peroxide-otic/article.htm
https://pubchem.ncbi.nlm.nih.gov/compound/Carbamide-Peroxide